Kariko and Weissman Study 2015: They discovered LNP/mRNA injection used during the development of the COVID-19 vaccines did not stay in the muscle, describing lipid distribution altered by injection.
Why weren't the COVID-19 mRNA vaccine consumers and providers warned that biodistribution was able to be significantly changed/manipulated via parameters of the injection/administration?
Below is an excerpt from my work on the lipids and LNPs in the mRNA vaccines that was sent to state AGs and other investigators this summer. Our states and other countries are investigating; why is our Federal government not investigating?
The full Executive Brief starts here The LNP Files..
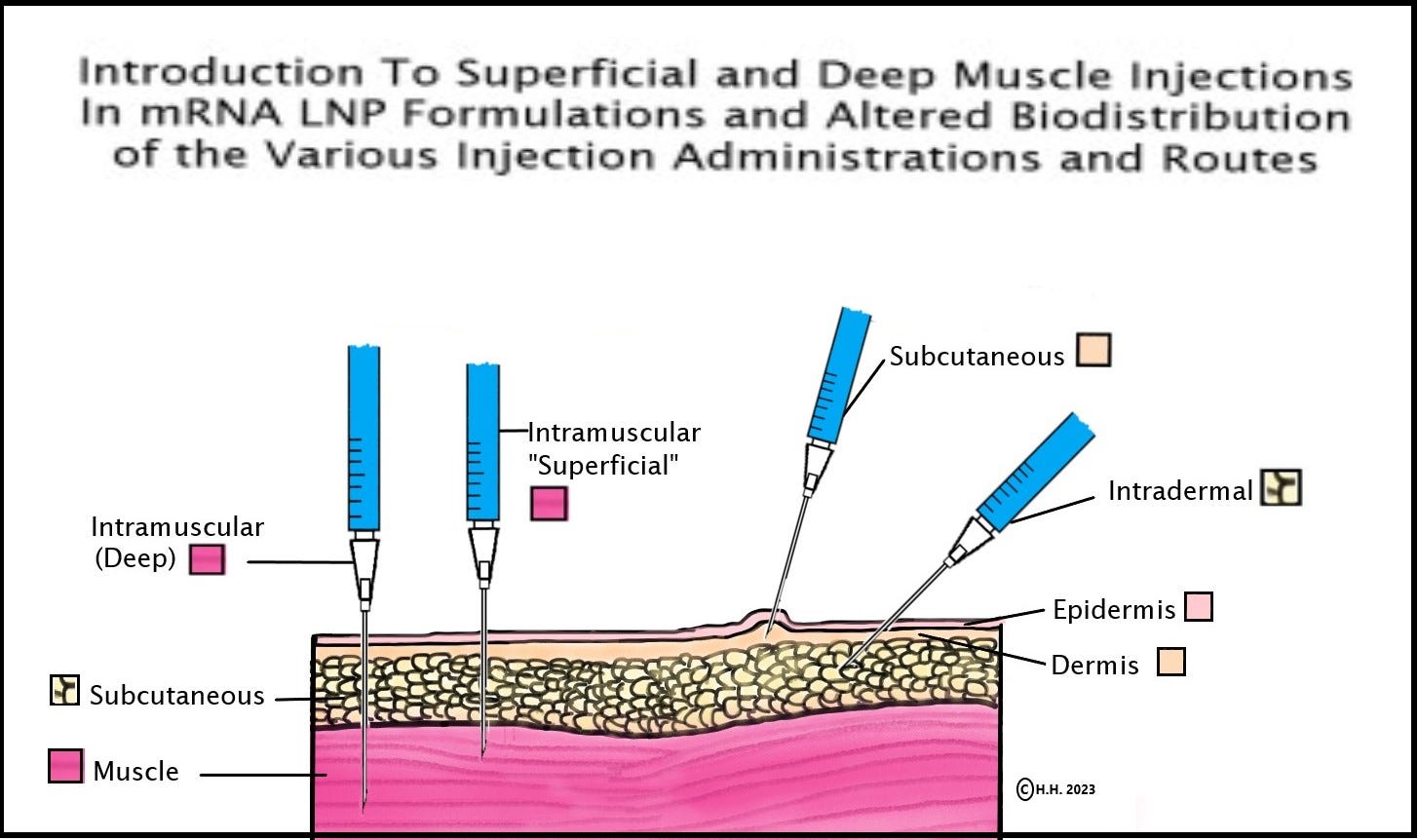
"In a 2015 animal study called “Expression Kinetics,” co-authored by a Senior V.P. of BioNTech Katalin Kariko and Drew Weissman (Developers for the Pfizer Covid-19 mRNA and developers of the Covid-19 mRNA-LNP vaccines) and two other authors, the study describes that the depth of the injection in the muscle for intramuscular mRNA-LNP formulations determines the organs/locations that the drug formula distributes to in the body, the amount of formula that is distributed to the location, and the length of time it remains in the body, as seen below:
a. “Here, we show that in vivo administration of mRNA-LNP complexes by various commonly used injection routes displays different expression patterns and kinetics. Depending on the site of injection, both local protein production and dissemination to the liver occurs. Interestingly, we observed that with intramuscular injection, the depth into the muscle also determined whether the majority of protein produced was in the muscle, for superficial injections, or in the liver, for deep muscular administration (unpublished observations). Importantly, very low doses (0.005 mg/kg) of mRNA-LNPs could be translated for several days following the tested delivery routes demonstrating the potential of these formulations for in vivo development.” [58] Again, this is written in 2015, co-authored by Weismann and Kariko.
b. The study shows that the location of the LNP injection (i.e. intramuscular, subcutaneous, or intratracheal) and, especially, the depth of the injection into the muscles shown to determine crucial outcomes as to where the LNPs (and mRNA) distribute in the body and organs. It also determines how much LNP (and mRNA) is distributed (into each location in the body and organs) and the amount of time the LNP remains in the body and organs.
c. Per the FDA, these factors are called biodistribution and pharmacokinetics. Their outcomes are used to determine safety and efficacy. They factor in the approval of a drug, even an EUA drug. In short, the “Expression Kinetics” animal study shows that the biodistribution and pharmacokinetics of LNP-mRNA formulations are variable and (for administration into the muscle) dependent on the depth of injection. [58]
d. The “Expression Kinetics” study also states, “Intramuscular (deep) and intratracheal delivery resulted in protein production both in the liver and at the injection sites… Protein production for intramuscular delivery, especially superficial injection, in the muscle could be measured for up to 8 days and high amounts of protein was made…” [58]
e. After reading about the Expression kinetics study, now consider the 2020 Wistar Han Rat Studies completed for the Pfizer Covid-19 mRNA-LNP vaccines, in which this information about the depth of the injection in the muscle is not given in the study or in any of the pre-clinical studies utilized in the Pfizer FDA EUA application or patient and provider information.
f. As we are told above in this study, the distribution and other outcomes of the mRNA-LNP studies are dependent upon the administration/injection site, and in the case of muscular injections (or intramuscular injections) –the outcome is dependent on the depth of the injection. [58] The details on the depth of injections given to the animals in the 2020 Pfizer pre-clinical animal models are not seen in the Pfizer Covid-19 mRNA vaccine studies provided to the FDA for Emergency Use Authorization (and later seen by consumers via FOIA). Without knowing or documenting the depth of the intramuscular injections of the mice/animals in the Covid-19 vaccine animal studies (as was done in the 2015 “Expression Kinetics” LNP-mRNA mouse study), the outcomes and variables of the Covid-19 LNP-mRNA vaccine animal models are missing these material factors that are shown to affect the outcome of LNP-mRNA mouse/animal biodistribution and safety studies. [58]
g. Please keep in mind that we are shown in the biodistribution studies that the LNP is meant to protect the mRNA as it travels in the body, it “carries” the mRNA (and the mRNA-LNP drugs are called LNP “carrier drugs”), and the LNP is found in the biodistribution into the liver and more for this reason. For example, in this 2021 study called, “Lipid nanoparticles for mRNA delivery, “ In addition to cationic or ionizable lipids, lipid nanoparticle–mRNA formulations typically contain other lipid components, such as phospholipids (for example, phosphatidylcholine and phosphatidylethanolamine), cholesterol or polyethylene glycol (PEG)-functionalized lipids (PEG-lipids)… These lipids can improve nanoparticle properties, such as particle stability, delivery efficacy, tolerability, and biodistribution.” [39][43]
h. Of importance, the provided FDA EUA patient fact sheet information and instructions on the administration of the Covid-19 mRNA-LNP vaccine injection in the Pfizer and Moderna Covid-19 vaccines show that practitioners and providers of Covid-19 mRNA vaccines were not given details on this vital “Expression Kinetics” study information on the depth of the intramuscular injection’s ability to alter mRNA-LNP drug delivery biodistribution and more. [25]
i. The “Expression Kinetics” study is discussed throughout this summary due to its importance: This vaccine administration information in the “Expression Kinetics” study was not included in the Pfizer FDA EUA Dosing and Administration instructions for medical providers. It was not mentioned in the biodistribution studies. However, the FDA guidelines for the novel COVID-19 EUA vaccine applicants, “Development and Licensure of Vaccines to Prevent COVID-19,” show the importance of the route of administration and possibility for tissue tropism and more, as seen here where it states, “These studies should be conducted if there is a likelihood of altered infectivity and tissue tropism or if a novel route of administration and formulation is to be used.” [78]
j. In biomedicine, according to the studies, cell tropism (tissue tropism) is altered or “enhanced” by the use of LNPs. Modifying the outcome of the LNPs also modifies tissue tropism or “cellular uptake” as seen here in the 2015 “Expression Kinetics” study which revealed that the variable depth of intramuscular route of administration for the mRNA-LNP formulation describe a modification of the mRNA-LNP delivery with an ability to impact cellular uptake and biodistribution. Yet, the importance and contents of this mRNA-LNP study are absent in Pfizer’s FDA EUA Covid-19 mRNA-LNP vaccine application and data. [58]
k. As further evidence and understanding of these studies, this 2018 article co-written by Drew Weissman (co-developer of the Pfizer Covid-19 vaccines), entitled “mRNA vaccines — a new era in vaccinology” states, “The magnitude and duration of in vivo protein production from mRNA–LNP vaccines can be controlled in part by varying the route of administration. Intramuscular and intradermal delivery of mRNA–LNPs has been shown to result in more persistent protein expression than systemic delivery routes...” [107] This information was not given to the consumer, and as we see here from item “a.” above, in the Expression Kinetics study, this information affects both the mRNA and the LNP “Importantly, very low doses (0.005 mg/kg) of mRNA-LNPs could be translated for several days following the tested delivery routes demonstrating the potential of these formulations for in vivo development.”
Were consumers told, and was the FDA told, that a study co-authored by parties that co-created the LNPs used in the Covid-19 mRNA-LNP vaccines --called “Expression Kinetics” study, written about mRNA-LNP kinetics-- reveals that LNP biodistribution (and more) are significantly variable and are able to be significantly changed/manipulated via parameters of the injection/administration of the drug into the muscle?"
2023 Heather Hudson
Please credit my work and link to this source if you repost any part of my work. Thank You
Click here: Full details with references can be found in my LNP Files Series, which starts here. The alarming LNP history you haven't been shown- The LNP developer's own studies dating back 20 years: Part 1 of 10, Introduction and Executive Brief sent to lawmakers & state investigators this summer.
About the author: Many may know me from my SubStack, media appearances, or testimony. I am the mother of Cody Hudson, who has a published medical case study and was significantly vaccine-injured at age 21. My research on the COVID-19 vaccine LNP has been published and is used in investigations into the COVID-19 mRNA vaccines. I write to bring awareness to these issues as I care for Cody. Please consider contributing to support my work or to help with his medical and educational expenses. Thank you.



Amazing work- thank you!
The "LNP files" page links to a private page which I was not able to access.
The "Expression Kinetics" rat study article is: Pardi, Tuyishime, Muramatsu, Kariko, Mui, Tam, Madden, Hope Drew Weissman, J Control Release, 2015-08-08 "Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes" https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4624045/ .
"When mRNA-LNPs were injected intramuscularly and intratracheally, similar to intravenous and intraperitoneal deliveries, a large portion of the luciferase activity was detectable in the liver, demonstrating systemic spread of the nanoparticles."
It may be difficult to reliably extrapolate from mice to humans, but in the absence of specific guidance for mRNA quasi-vaccine injection technique, and especially the lack of requirement of aspirating the needle to reduce the chance that the fluid is injected into a blood vessel, it is reasonable to expect that this uncontrolled injection regime would result in significant delivery of the lipid nanoparticle contents to distant parts of the body, including the liver and potentially all other organs, especially the heart.
Also of interest is this 2023-10-11 article https://www.mdpi.com/2076-393X/11/10/1580 in which mice given intramuscular injections of LNPs containing mRNA OR DNA plasmids encoding a gene which, when expressed to create protein, causes the cell to glow. The LNPs were made with different formulations.
"Following intramuscular injection in mice, we determined that RNA-LNPs formulated with either SM-102 or ALC-0315 lipids were the most potent (all p-values < 0.01) and immunogenic (all p-values < 0.05), while DNA-LNPs formulated with SM-102 or ALC-0315 demonstrated the longest duration of signal. Additionally, all LNP formulations were found to induce expression in the liver that was proportional to the signal at the injection site . . ."
Since we can't rule out the possibility that human quasi-vaccine injections, at least in some instances, were functionally intramuscular, we can reasonably assume that their intended mRNA (modified messenger RNA) payloads, and their contaminants (fragments and/or erroneous mRNA and fragments of DNA) will be delivered to and alter the function of cells in many and perhaps all parts of the body often enough to cause effects which were not intended by the vaccine designers.
I found this page via: https://phillipaltman.substack.com/p/shocking-revelations-on-the-covid